Healthcare Professionals

Healthcare Professionals
Paediatric Reference Intervals
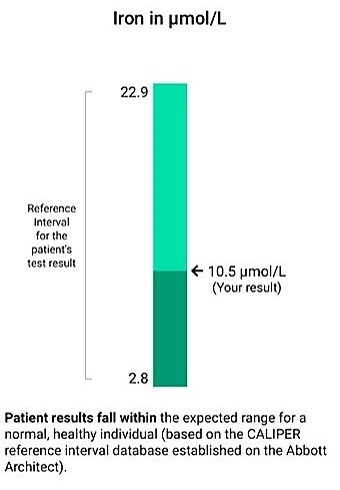
Reference intervals, also known as reference standards, can be defined as health-associated benchmarks that are essential to clinical decision-making. Reference intervals are widely used by clinical laboratories to flag results, thereby notifying clinicians of the potential need for follow up, additional tests, specialist referral, and/or treatment. Statistically, reference intervals are represented as the 2.5th and 97.5th percentiles derived from blood test results obtained in a healthy reference population. There are two main approaches to calculate a reference interval:
Direct: Sampling from healthy volunteers with informed consent followed by sample and data analysis for laboratory test of interest in alignment with Clinical and Laboratory Standards Institute (CLSI) guidelines.
Indirect: Use of retrospective laboratory data collected as part of patient care followed by the application of complex statistical algorithms (e.g. Bhattacharya, Hoffmann, Truncated Maximum Likelihood)
While the direct approach is considered the gold standard, it requires significant resources and presents challenges for many clinical laboratories. In contrast, indirect statistical methods require minimal resources, relying solely on statistical techniques to ‘mine’ for healthy test result values in a patient dataset. Indirect statistical methods have significantly improved in recent years with many studies supporting its application clinically. If you are interested in learning more on indirect methods, consider reading the International Federation of Clinical Chemistry (IFCC) Committee on Reference Intervals and Decision Limits (C-RIDL) review and recommendations for indirect methods for reference interval determination.
Children are not small adults!
Given the critical importance to healthcare, it would be expected that comprehensive reference intervals are available and implemented in clinical institutions worldwide, but this is not always the case – particularly in paediatrics. Rapid physiological growth and development throughout childhood and adolescence influences biochemistry, necessitating age- and sex-specific interpretation. For example, alkaline phosphatase, a biomarker of liver disease, varies significantly from birth to adolescence due to changes in muscle mass, particularly in males, as well as kidney maturation associated with development. The use of a reference interval developed in adults for this biomarker in children and adolescents would result in gross over-flagging and inappropriate follow up. This is true for many biomarkers, highlighting the need for paediatric reference intervals.
Who can participate?
- Healthy children (determined based on questionnaire responses)
- Children who are 0 to 18 years old
Who cannot participate?
- Children with a history of chronic illness or metabolic disease
- Children who have had an acute illness within the past month
- Children who have taken prescribed medication in the past month.
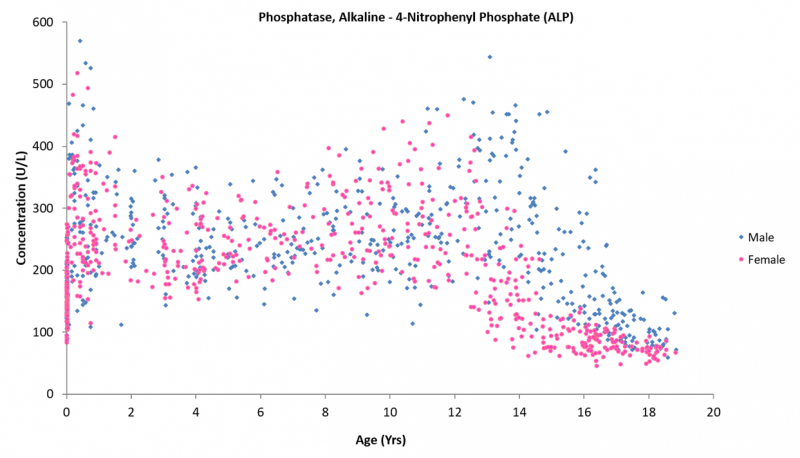
Until recently, a major gap existed in the availability of paediatric reference intervals for common laboratory tests. As a result, laboratories and healthcare professionals relied on adult reference intervals or incomplete data as a source of paediatric reference intervals. This increases the risk of misdiagnosis and can result in unnecessary stress, lengthier hospital stays, and increased costs to the health-care system. CALIPER, as well as a number of other initiatives, has made significant progress in establishing paediatric reference intervals. Despite this, gaps still exist in paediatric reference intervals and the evaluation of paediatric test results. We’re making a push to fill these gaps, alongside a number of other initiatives.
CALIPER Program Design
Cohort recruitment
Children from birth to 18 years of age are eligible to take part in CALIPER, upon written informed consent. Through a community-based approach, we hold events at schools, universities, community centers, and summer camps to provide opportunities for children across the Toronto area to participate. Eligibility is determined by self-reported questionnaire, requesting information on history of chronic and acute illness, prescribed and non-prescribed medication use, diet, and ethnicity. Participants are excluded if they report: 1) a history of chronic illness or metabolic disease, 2) acute illness within past month, and 3) use of prescribed medication within the past month.
Who can participate?
- Healthy children (determined based on questionnaire responses)
- Children who are 0 to 18 years old
Who cannot participate?
- Children with a history of chronic illness or metabolic disease
- Children who have had an acute illness within the past month
- Children who have taken prescribed medication in the past month.
Sample collection and analysis
Who can participate?
- Healthy children (determined based on questionnaire responses)
- Children who are 0 to 18 years old
Who cannot participate?
- Children with a history of chronic illness or metabolic disease
- Children who have had an acute illness within the past month
- Children who have taken prescribed medication in the past month.
Depending on the age of the child, one to 11 mL of whole blood is collected in Serum Separator Tubes (SST, BD) by trained paediatric phlebotomists using standardized conditions. Specific collection tubes may also be used depending on the laboratory test of interest (e.g. KEDTA, LiHep, NaCitrate, Royal Blue Top). Blood samples are centrifuged at 4000 rpm for 10 minutes within eight hours of collection, aliquoted into 0.5 mL cryovials, and stored at –80°C. All samples are de-identified prior to storage.
Samples have been analyzed on several analytical platforms, including analyzers from Abbott Diagnostics, Ortho Clinical Diagnostics, DiaSorin, Roche Diagnostics, Beckman Coulter Diagnostics, Siemens Healthineers, as well as Liquid Chromatography and Tandem Mass Spectrometry (LC-MS/MS), Inductively Coupled Plasma Mass Spectrometry (ICP-MS), and High-Performance Liquid Chromatography (HPLC) techniques. Analytical methods are controlled according to manufacturers’ instructions via preventive maintenance and function checks, calibration, and quality control. All tests are subjected to automated interference analysis for hemolysis, icterus, and turbidity.
Who can participate?
- Healthy children (determined based on questionnaire responses)
- Children who are 0 to 18 years old
Who cannot participate?
- Children with a history of chronic illness or metabolic disease
- Children who have had an acute illness within the past month
- Children who have taken prescribed medication in the past month.


Data analysis and statistics
We use a highly standardized direct approach to establishing all reference intervals. They’re calculated based on guidelines published by the Clinical Laboratory Standards Institute (CLSI) in their report Defining, Establishing, and Verifying Reference Intervals in the Clinical Laboratory; Approved Guideline – Third Edition (C28-A3).
In summary:
- Data is visually examined to determine appropriate age and sex partitions. Partitions are then statistically evaluated using the Harris and Boyd method.
- Outliers are removed from each age and sex partition using the Tukey or Adjusted Tukey method, based on data distribution.
- Non-parametric or robust methods are used to determine specific reference intervals. For partitions with sample sizes >120, the non-parametric rank method is used, while the robust statistical methods are applied to partitions <120. The 90 per cent confidence intervals around the lower and upper reference limits are then calculated.
All statistical analysis is performed using MS Excel, SPSS, and R software.
For a more detailed description of the methods we apply when calculating intervals, and then transferring those values to other instruments, refer to our list of publications or contact us at caliper.project@sickkids.ca.
CALIPER Studies
We continue to establish accurate paediatric reference intervals for all routine and specialized biomarkers of health and disease across several different laboratory testing systems. This ensures laboratories, healthcare professionals, and families have access to robust reference intervals, regardless of the type of equipment used to test children and adolescents. Our team is also currently focused on sub-studies regarding maternal health, early childhood, COVID-19, and paediatric obesity. If you are interested in learning more, please find additional information below or contact us!




Publications
Highlighted publications
- Adeli K, Higgins V, Trajcevski K, White-Al Habeeb N. The Canadian laboratory initiative on pediatric reference intervals: A CALIPER white paper. Crit Rev Clin Lab Sci. 2017 Sep;54(6):358-413. doi: 10.1080/10408363.2017.1379945.
- Adeli K, Higgins V, Trajcevski K, Palmert MR. Important considerations for interpreting biochemical tests in children. BMJ. 2018 May 24;361:k1950. doi: 10.1136/bmj.k1950.
- Higgins V, Asgari S, Hamilton JK, Wolska A, Remaley AT, Hartmann B, Holst JJ, Adeli K. Postprandial dyslipidemia, hyperinsulinemia, and impaired gut peptides/bile acids in adolescents with obesity. J Clin Endocrinol Metab. 2020 Apr 1; 105(4):dgz261. doi: 10.1210/clinem/dgz261.
- Higgins V, Omidi A, Tahmasebi H, Asgari S, Gordanifar K, Nieuwesteeg M, Adeli K. Marked influence of adiposity on laboratory biomarkers in a healthy cohort of children and adolescents. J Clin Endocrinol Metab. 2020 Apr 1;105(4):dgz161. doi: 10.1210/clinem/dgz161.
- Tahmasebi H, Asgari S, Hall A, Higgins V, Chowdhury A, Thompson R, Bohn MK, Macri J, Adeli K. Influence of ethnicity on biochemical markers of health and disease in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med. 2020 Mar 26;58(4):605-617. doi: 10.1515/cclm-2019-0876.
Recent publications
- Badakhshan SN, Ghazizadeh H, Mohammadi-Bajgiran M, et al. Age-specific reference intervals for liver function tests in healthy neonates, infants, and young children in Iran. J Clin Lab Anal. 2023;37(23-24):e24995. doi:10.1002/jcla.24995
- Barakauskas VE, Bohn MK, Branch E, et al. Mining the Gap: Deriving Pregnancy Reference Intervals for Hematology Parameters Using Clinical Datasets. Clin Chem. 2023;69(12):1374-1384. doi:10.1093/clinchem/hvad167
- Stukas S, Cooper J, Higgins V, Holmes D, Adeli K, Wellington CL. Pediatric reference intervals for serum neurofilament light and glial fibrillary acidic protein using the Canadian Laboratory Initiative on Pediatric Reference Intervals (CALIPER) cohort. Clin Chem Lab Med. 2023;62(4):698-705. Published 2023 Oct 27. doi:10.1515/cclm-2023-0660
- Miller JJ, Bohn MK, Higgins V, et al. Pediatric reference intervals for endocrine markers in healthy children and adolescents on the Liaison XL (DiaSorin) immunoassay system. Clin Biochem. 2023;120:110644. doi:10.1016/j.clinbiochem.2023.110644
- Bohn MK, Steele S, Adeli K. SARS-CoV-2 serology in pediatrics: Seroprevalence studies in unvaccinated children and humoral antibody response post vaccination. Clin Biochem. 2023;119:110630. doi:10.1016/j.clinbiochem.2023.110630
- Mohammadi M, Ghazizadeh H, Mohammadi-Bajgiran M, et al. Pediatric reference intervals for hematology parameters in healthy infants and young children in Iran. Int J Lab Hematol. 2023;45(6):845-852. doi:10.1111/ijlh.14132
- Bohn MK, Nichols M, Yang L, Bhayana V, Macri J, Adeli K. Pediatric Reference Value Profiling of Essential Trace and Toxic Elements in Healthy Children and Adolescents Using High-Resolution and Triple Quadrupole Inductively Coupled Plasma Mass Spectrometry. J Appl Lab Med. 2023;8(4):674-688. doi:10.1093/jalm/jfad019
- Bohn MK, Schneider R, Jung B, Adeli K. Pediatric reference interval verification for 16 biochemical markers on the Alinity ci system in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med. 2023;61(11):2033-2040. Published 2023 May 1. doi:10.1515/cclm-2023-0256
- Di Meo A, Yazdanpanah M, Higgins V, et al. Highly sensitive tandem mass spectrometric measurement of serum estradiol without derivatization and pediatric reference intervals in children and adolescents. Clin Chem Lab Med. 2023;61(10):1820-1828. Published 2023 Apr 11. doi:10.1515/cclm-2022-1231
- Ghazizadeh H, Kathryn Bohn M, Esmaily H, et al. Comparison of reference intervals for biochemical and hematology markers derived by direct and indirect procedures based on the Isfahan cohort study. Clin Biochem. 2023;116:79-86. doi:10.1016/j.clinbiochem.2023.04.001
- Bohn MK, Adeli K. Comprehensive Pediatric Reference Limits for High-Sensitivity Cardiac Troponin I and NT-proBNP in the CALIPER Cohort. J Appl Lab Med. 2023;8(3):443-456. doi:10.1093/jalm/jfad012
- Bohn MK, Wilson S, Steele S, Adeli K. Comprehensive pediatric reference intervals for 79 hematology markers in the CALIPER cohort of healthy children and adolescents using the Mindray BC-6800Plus system. Int J Lab Hematol. 2023;45(4):469-480. doi:10.1111/ijlh.14068
- Wilson SM, Bohn MK, Madsen A, Hundhausen T, Adeli K. LMS-based continuous reference percentiles for 14 laboratory parameters in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med. 2023;61(6):1105-1115. Published 2023 Jan 16. doi:10.1515/cclm-2022-1077
- Abdollahian N, Ghazizadeh H, Mohammadi-Bajgiran M, Pashirzad M, Yaghooti Khorasani M, Bohn MK, Steele S, Roudi F, Kamel Khodabandeh A, Ghazi Zadeh S, Alami-Arani I, Badakhshan SN, Esmaily H, Ferns GA, Assaran-Darban R, Adeli K, Ghayour-Mobarhan M. Age-specific reference intervals for routine biochemical parameters in healthy neonates, infants, and young children in Iran. J Cell Mol Med. 2023 Jan;27(1):158-162. doi: 10.1111/jcmm.17646.
- Haeckel R, Adeli K, Jones G, Sikaris K, Wosniok W. Definitions and major prerequisites of direct and indirect approaches for estimating reference limits. Clin Chem Lab Med. 2022 Dec 2;61(3):402-406. doi: 10.1515/cclm-2022-1061.
- Olver P, Bohn MK, Adeli K. Central role of laboratory medicine in public health and patient care. Clin Chem Lab Med. 2022 Nov 28;61(4):666-673. doi: 10.1515/cclm-2022-1075.
- Bohn MK, Wilson S, Schneider R, Massamiri Y, Randell EW, Adeli K. Pediatric reference interval verification for 17 specialized immunoassays and cancer markers on the Abbott Alinity i system in the CALIPER cohort of healthy children and adolescents. Clin Chem Lab Med. 2022 Sep 19;61(1):123-132. doi: 10.1515/cclm-2022-0709.
- Sepiashvili L, Bohn MK, Hall A, Henderson T, Chen J, Dunst R, Adeli K. Determination of pediatric reference limits for 10 commonly measured autoantibodies. Clin Chem Lab Med. 2022 Sep 19;60(11):1839-1846. doi: 10.1515/cclm-2022-0675.
- Ronn R, Bohn MK, Greenblatt EM, Hoffman B, Adeli K. Anti-Mullerian Hormone (AMH) reference values in the CALIPER cohort of healthy community children and adolescent. Clin Biochem. 2022 Oct;108:63-6. doi: 10.1016/j.clinbiochem.2022.06.005.
- Wilson S, Earle H, Bohn MK, Hall A, Adeli K. Pediatric Reference Intervals for Point-of-Care Random Glucose in Healthy Children and Adolescents. J Appl Lab Med. 2022 Mar 2;7(2):582-88. doi: 10.1093/jalm/jfab155.
- He F, Yang R, Huang X, Tian Y, Pei X, Bohn MK, Zou L, Wang Y, Li H, Wang T, Gu M, Jiang T, Chen X, Zou H, Wei H, Tian W, Tang T, Adeli K, Wang Z. Reference Standards for Newborn Screening of Metabolic Disorders by Tandem Mass Spectrometry: A Nationwide Study on Millions of Chinese Neonatal Populations. Front Mol Biosci. 2021 Dec 16;8:719866. doi: 10.3389/fmolb.2021.719866.
- Bohn MK, Horn P, League D, Steele P, Hall A, Adeli K. Pediatric reference intervals for 32 routine biochemical markers using the siemens healthineers atellica® CH assays in healthy children and adolescents. Clin Biochem. 2022 Jan;99:69-77. doi: 10.1016/j.clinbiochem.2021.10.006.
- Bohn MK, Adeli K. Physiological and metabolic adaptations in pregnancy: importance of trimester-specific reference intervals to investigate maternal health and complications. Crit Rev Clin Lab Sci. 2022 Mar;59(2):76-92. doi: 10.1080/10408363.2021.1978923.
- Sepiashvili L, Alli Z, Bohn MK, Hall A, Karin A, Murata K, Adeli K. Complex biological patterns of soluble cytokines and CD136 in childhood necessitating age-specific reference intervals for evidence-based clinical interpretation. Clin Biochem. 2021 Dec;98:35-41. doi: 10.1016/j.clinbiochem.2021.09.004.
- Bohn MK, Yousef P, Steele S, Sepiashvili L, Adeli K. Multi-Inflammatory Syndrome in Children: A View into Immune Pathogenesis from a Laboratory Perspective. J Appl Lab Med. 2022 Jan 5;7(1):311-21. doi: 10.1093/jalm/jfab114.
- Wilson S, Bohn MK, Adeli K. POCT: An Inherently Ideal Tool in Pediatric Laboratory Medicine. EJIFCC. 2021 Jun 29;32(2):145-157.
- Wilson S, Bohn MK, Hall A, Higgins V, Abdelhaleem M, Adeli K. Continuous reference curves for common hematology markers in the CALIPER cohort of healthy children and adolescents on the Sysmex XN-3000 system. Int J Lab Hematol. 2021 Dec;43(6):1394-1402. doi: 10.1111/ijlh.13670.
- Bohn MK, Wilson S, Hall A, Adeli K. Pediatric reference interval verification for endocrine and fertility hormone assays on the Abbott Alinity system. Clin Chem Lab Med. 2021 Jun 30;59(10):1680-1687. doi: 10.1515/cclm-2021-0337.
- Bohn MK, Steele S, Hall A, Poonia J, Jung B, Adeli K. Cardiac Biomarkers in Pediatrics: An Undervalued Resource. Clin Chem. 2021 Jul 6;67(7):947-958. Review. doi: 10.1093/clinchem/hvab063.
- Bohn MK, Hall A, Wilson S, Henderson T, Adeli K. Pediatric Reference Intervals for Critical Point-of-Care Whole Blood Assays in the CALIPER Cohort of Healthy Children and Adolescents. Am J Clin Pathol. 2021 Nov 8;156(6):1030-1037. doi: 10.1093/ajcp/aqab064.
- Hamid JS, Atenafu EG, Borkhoff CM, Birken CS, Maguire JL, Bohn MK, Adeli K, Abdelhaleem M, Parkin PC. Reference intervals for hemoglobin and mean corpuscular volume in an ethnically diverse community sample of Canadian children 2 to 36 months. BMC Pediatr. 2021 May 19;21(1):241. doi: 10.1186/s12887-021-02709-w.
- Bohn MK, Wilson S, Hall A, Massamiri Y, Randell E, Adeli K. Pediatric reference interval verification for common biochemical assays on the Abbott Alinity system. Clin Chem Lab Med. 2021 May 19;59(9):1554-1562. doi: 10.1515/cclm-2021-0336.
- Bohn MK, Horn P, League D, Steele P, Hall A, Adeli K. Pediatric reference intervals for endocrine markers and fertility hormones in healthy children and adolescents on the Siemens Healthineers Atellica immunoassay system. Clin Chem Lab Med. 2021 May 7;59(8):1421-1430. doi: 10.1515/cclm-2021-0050.
- Hall A, Bohn MK, Wilson S, Higgins V, Adeli K. Continuous reference intervals for 19 endocrine, fertility, and immunochemical markers in the CALIPER cohort of healthy children and adolescents. Clin Biochem. 2021 Aug;94:35-41. doi: 10.1016/j.clinbiochem.2021.04.014.
- Bohn MK, Hall A, Wilson S, Taher J, Sepiashvili L, Adeli K. Pediatric evaluation of clinical specificity and sensitivity of SARS-CoV-2 IgG and IgM serology assays. Clin Chem Lab Med. 2021 Jan 22;59(6):e235-e237. doi: 10.1515/cclm-2020-1822.
- Lam E, Higgins V, Zhang L, Chan MK, Bohn MK, Trajcevski K, Liu P, Adeli K, Nathan PC. Normative values of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in children and adolescents: A study from the CALIPER cohort. J Appl Lab Med. 2021 Mar 1;6(2):344-353. doi: 10.1093/jalm/jfaa090.
- Bohn MK, Higgins V, Tahmasebi H, Hall A, Liu E, Adeli K, Abdelhaleem M. Complex biological patterns of hematology parameters in childhood necessitating age- and sex-specific reference intervals for evidence-based clinical interpretation. Int J Lab Hematol. 2020 Dec;42(6):750-760. doi: 10.1111/ijlh.13306.
- Thompson S, Bohn MK, Mancini N, Loh TP, Wang CB, Grimmler M, Yuen KY, Mueller R, Koch D, Sethi S, Rawlinson WD, Clementi M, Erasmus R, Leportier M, Kwon GC, Menezes ME, Patru MM, Gramegna M, Singh K, Najjar O, Ferrari M, Lippi G, Adeli K, Horvath A and the IFCC Taskforce on COVID-19. IFCC interim guidelines on biochemical/hematological monitoring of COVID-19 patients. Clin Chem Lab Med. 2020 Oct 7;58(12):2009-2016. doi: 10.1515/cclm-2020-1414.
- Bohn MK, Loh TP, Wang CB, Mueller R, Koch D, Sethi S, Rawlinson WD, Clementi M, Erasmus R, Leportier M, Grimmler M, Yuen KY, Mancini N, Kwon GC, Menezes ME, Patru MM, Gramegna M, Singh K, Najjar O, Ferrari M, Horvath A, Lippi G, Adeli K and the IFCC Taskforce on COVID-19. IFCC interim guidelines on serological testing of antibodies against SARS-CoV-2. Clin Chem Lab Med. 2020 Oct 7;58(12):2001-2008. doi: 10.1515/cclm-2020-1413.
- Bohn MK, Mancini N, Loh TP, Wang CB, Grimmler M, Gramegna M, Yuen KY, Mueller R, Koch D, Sethi S, Rawlinson WD, Clementi M, Erasmus R, Leportier M, Kwon GC, Menezes ME, Patru MM, Singh K, Ferrari M, Najjar O, Horvath A, Adeli K, Lippi G and the IFCC Taskforce on COVID-19. IFCC interim guidelines on molecular testing of SARS-CoV-2 infection. Clin Chem Lab Med. 2020 Oct 7;58(12):1993-2000. doi: 10.1515/cclm-2020-1412.
- Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity Progression. Physiology (Bethesda). 2020 Sep 1;35(5):288-301. doi: 10.1152/physiol.00019.2020.
- Higgins V, Tahmasebi H, Bohn MK, Hall A, Adeli K. CALIPER Hematology Reference Standards (I). Am J Clin Pathol. 2020 Aug 5;154(3):330-341. doi: 10.1093/ajcp/aqaa059.
- Higgins V, Tahmasebi H, Bohn MK, Hall A, Adeli K. CALIPER Hematology Reference Standards (II). Am J Clin Pathol. 2020 Aug 5;154(3):342-352. doi: 10.1093/ajcp/aqaa057.
- Adeli K. Critical role of laboratory medicine in the global response to the COVID-19 pandemic. Clin Chem Lab Med. 2020 Jun 25;58(7):1019-1020. doi: 10.1515/cclm-2020-0742.
- Bohn MK, Lippi G, Horvath A, Sethi S, Koch D, Ferrari M, Wang CB, Mancini N, Steele S, Adeli K. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation and latest evidence. Clin Chem Lab Med. 2020 Jun 25;58(7):1037-1052. doi: 10.1515/cclm-2020-0722.
- Higgins V, Adeli K. Postprandial dyslipidemia in insulin resistant states in adolescent populations. J Biomed Res. 2020 Jan 30;34(5):328-342. doi: 10.7555/JBR.34.20190094.
Resources
CALIPER has established paediatric intervals, partitioned according to age and sex, for a number of common biochemical, immunological, hematological, nutritional, endocrine, and fertility markers, as well as many special chemistry evaluations, on a variety of different testing platforms that are available on the CALIPER database and mobile app. As the CALIPER program continues to grow and develop, the database will be updated with new tests.
You can refer to the CALIPER database by following the link below for the latest updates.
And don’t forget to download the CALIPER app, available for both iOS and Android devices! It’s designed for paediatricians, family physicians, healthcare workers, and others like yourself. This intuitive, easy-to-use tool can help healthcare professionals assess a child’s laboratory test results using the latest reference intervals based on values in healthy children and adolescents.


